- Startseite
- Blog
- Corrosion vs Oxidation vs Rust: Detailed Comparison
Corrosion vs Oxidation vs Rust: Detailed Comparison
Corrosion, oxidation, and rust are frequently sound the equal. Each has special sources and results on materials. Understanding these variations is significant for engineers, industries, and even in daily life.
In this blog, you can learn about corrosion vs oxidation vs rust in depth, including processes, examples, industrial effects, and prevention methods.
What is Oxidation?
Oxidation is a chemical reaction where a material loses its electrons, often including oxygen. It is not constantly dangerous, but it plays a primary role in natural and industrial processes.
For example, oxidation is necessary in cellular respiration, combustion, and metal reactions. At the same time, several oxidations are helpful in metals. It can decrease strength and create long-term issues. It is an extensive term that fits both metals and non-metals.
Chemical Reaction Process of Oxidation

Oxidation happens when atoms or molecules donate electrons. In metals, oxygen atoms bond with metal atoms, creating oxides. For example, when iron responds with oxygen in the air, it makes iron oxide. In burning, oxidation discharges energy, that’s why fuels burn.
| Oxidation Type | Example | Result |
| Metal Oxidation | Iron + O₂ → Iron Oxide | Directs to rust in the appearance of humidity. |
| Combustion | Hydrocarbon + O₂ → CO₂ + H₂O | Generates heat/energy. |
| Biological | Glucose oxidation in cells | Powers metabolism |
This method can either be beneficial, such as energy production, or harmful, such as the reduction of metals.
Common Examples of Oxidation in Daily Life
Rusting of Iron and Steel

When iron or steel is exposed to oxygen and humidity, this is a slow process, but it leads to a critical reduction of the material. It is frequent in bridges, vehicles, and household instruments. The oxide coating is brittle and does not protect the metal below.
Tarnishing of Silver
Silver does not rust, but it reacts with sulfur compounds in the air. This makes a black layer called tarnish. While it does not smash silver totally, it damages its look and needs shining or buffing. Unlike rust, tarnish can be eliminated smoothly and does not extend extra once purified.
What is Corrosion?
Corrosion is the destruction of metals due to reactions with surrounding elements like oxygen, salts, etc. It is more detrimental than general oxidation since it creates framework wastes.
Unlike oxidation, corrosion is mostly harmful and needs continuous protection from it. It is a true challenge in every branch of engineering.
Types of Corrosion
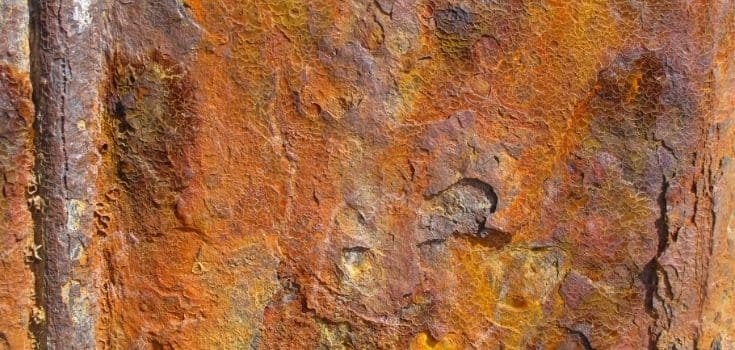
Galvanic Corrosion
When two unlike metals are linked together in any electrolyte, such as water, one of them becomes the anode and suffers more rapid corrosion, while the other is protected.
For instance, when steel is joined to copper, its corrosion may become very rapid due to the flow of electrons from steel to copper.
Pitting Corrosion
This is a localized form of corrosion showing small holes on a metal surface. It is dangerous, for it can lead to deep penetration into the structure, giving rise to unexpected equipment failure. Pitting is broadly associated with chloride-rich environments like seawater.
Crevice Corrosion
This happens at hidden locations where water or dirt may accumulate, such as joints, seals, and overlaps. Because it is hard to detect, it can damage machinery and parts without giving any warning. Usually, it occurs in stagnant or low-oxygen zones.
Materials Susceptible to Corrosion
| Material | Susceptibility | Examples of Use |
| Iron & Steel | Hoch | Construction, vehicles, tools |
| Aluminium | Mittel | Aerospace, packaging, roofing |
| Kupfer | Mittel | Electrical wires, plumbing |
| Rostfreier Stahl | Niedrig | Surgical tools, appliances |
Different metals provide different corrosion actions. Selecting the right material for an environment is essential in product creation.
Industrial Impact of Corrosion

Corrosion is costly. It has extremely high costs for industries every single year.
- In oil and gas, corrosion reduces pipes, causing leakages that present safety risks.
- In automotive and aerospace, corrosion affects the life reduction of the vehicle and higher maintenance costs.
- Construction projects can collapse when the steel supports rust.
| Industrie | Corrosion Impact |
| Öl und Gas | Pipes, explosions, and environmental damage |
| Automobilindustrie | Vehicle body rust, structural failure |
| Luft- und Raumfahrt | Reduced airframe integrity, safety hazards |
| Infrastructure | Bridge collapse risk, building degradation |
Difference Between Oxidation and Corrosion

Oxidation is a chemical reaction that can take place in the presence or the absence of oxygen. Any process involved in the modification of materials is not damaging. On the contrary, corrosion actually refers to the destruction of metals as a result of oxidation and other reactions.
| Aspekt | Oxidation | Corrosion |
| Definition | Loss of electrons during a reaction | Degradation of metal by environmental factors |
| Materialien | Metals and non-metals | Only metals |
| Impact | Can be useful or harmful | Always harmful |
| Examples | Combustion, rusting, tarnishing | Rust, pitting, galvanic corrosion |
What is Rust?
The most popular example of corrosion is rust. It only occurs in Eisen und Stahl. The reddish-brown coating appears on surfaces. Rust reduces the strength of the metal, causing breakable formation and spreading quickly, especially in moist environments.
Rust does not occur on aluminum or copper. Their surface oxides are different and sometimes can be protective.
Oxidation Reaction
Rust is a result of an oxidation reaction in which iron atoms lose electrons to oxygen in the presence of water. Hydrated iron oxides are created during this process, which flakes off to expose new metallic surfaces, continuously damaging the metal.
Conditions That Lead to Rust

- Moisture is present all the time
- Contacts with oxygen in the atmosphere
- Salt or humid exposure
- Temperature fluctuation that leads to condensation
These factors show the processes of rusting. Rusting is much more rapid in outdoor equipment than in indoor equipment.
Commonly Affected Materials
Corrosion mainly attacks iron & steel. Once corrosion starts, it spreads if not treated quickly.
| Material | Gemeinsame Anwendungen |
| Eisen | Pipes, structural components, cookware |
| Stahl | Bridges, ships, tools, vehicles |
Alloys like stainless steel are designed with chromium to resist rust corrosion and form a protective oxide layer.
How to Prevent Rust?
- Coatings like paint or powder coating should be applied.
- Stainless steel or alloys with chromium should be employed.
- Store in dry conditions and maintain moisture-free contact.
- Rust inhibitors should be applied in pipelines and industrial systems.
| Method | How It Helps |
| Painting or coating | It creates a physical barrier between air and water. |
| Verzinkung | Zinc is used to protect the steel. |
| Rostfreier Stahl | Chromium forms a passive oxide layer |
| Storage | It reduces exposure to moisture in the storage environments |
Key Differences between Corrosion vs Oxidation vs Rust
Here are the key differences between corrosion, Oxidation, and Rust:
| Term | Meaning | Materials Affected | Effect on Material |
| Oxidation | Electron loss in a reaction | Metals & non-metals | May be useful or damaging |
| Corrosion | Degradation of metals due to the environment | Metalle | Weakening of the structure |
| Rust | Corrosion of iron and steel to form iron oxide | Iron, steel | Reddish brown, flaky, brittle, spreads extremely fast |
Schlussfolgerung
All three, corrosion, oxidation, and rust, may overlap in some moments, but each has its own causes and effects. Knowing their differences will help protect some materials against damage while improving their long life.
DEK helps you with advanced machining and protective treatments to fight corrosion and rust. Kontakt to know more about durable solutions for your projects.
